BactoSiA by OptraSCAN® combines high-resolution brightfield whole-slide scanning with trained AI algorithms to automatically detect and quantify acid-fast bacilli (AFB) on Ziehl–Neelsen-stained sputum samples. This end-to-end solution is designed to make TB detection more efficient, accessible, and actionable, even in low-resource settings with limited on-site expertise. BactoSiA is powered by OptraSCAN's OS-SiA™ technology - the world's first patented technology that scans, indexes, and analyzes simultaneously.
Tuberculosis remains a significant global health challenge, particularly in regions where laboratories must manage large testing volumes using smear microscopy due to accessibility, infrastructure, and cost considerations.
As demand for TB testing continues, laboratories face increasing pressure to process more samples efficiently, maintain consistency across readers, and reduce manual workload, all while preserving established laboratory practices.
There is a clear need to modernize smear microscopy workflows to support faster, more standardized detection, monitoring, and follow-up without increasing operational burden.
BactoSiA enhances routine smear microscopy by combining intelligent slide digitization, AI assisted analysis, and digital review into a single, integrated system.
BactoSiA is powered by OS-SiA™ technology - the world’s first patented technology by OptraSCAN that scans, indexes, and analyzes simultaneously in minutes.
Using OptraSCAN scanners powered by OS-SiA™ technology, slides are digitized, indexed, and analyzed as part of the scanning process, enabling laboratories to gain efficiency directly at the point of acquisition rather than relying on downstream processing.
Scan. Index. Analyze.
Scan:
Smear slides are prepared using standard laboratory protocols and processed on OptraSCAN systems (OS-SiX™, OS-Lite™, OS-Ultra™, and OS-FLi™) to generate high-resolution digital slide images for analysis.
Index:
Automatically generates indexed thumbnails, slide maps, metadata,and contextual information. Once the scan is complete, the slides are already categorized, cataloged, and made searchable. Each scanned region is instantly tagged, and database is prepared for easy integration into the lab workflow
Analyze:
During acquisition, BactoSiA AI performs automated slide triage, marks FOVs, and triages slides as "likely negative" or "likely positive" to prioritize pathologist review. Confirmed positive smears are graded using standardized criteria aligned with WHO guidelines. All images and reports are managed digitally in IMAGEPath® software
BactoSiA™ is designed to support a seamless transition from slide preparation through digital review within routine smear microscopy workflows. By integrating scanning, indexing, and AI-assisted analysis into a single process, slides are categorized as likely positive or likely negative to support structured review prioritization, with positive cases highlighted for focused assessment. This integrated workflow helps laboratories manage testing demand, reduce manual handling, and maintain consistent evaluation practices while operating within established laboratory processes.
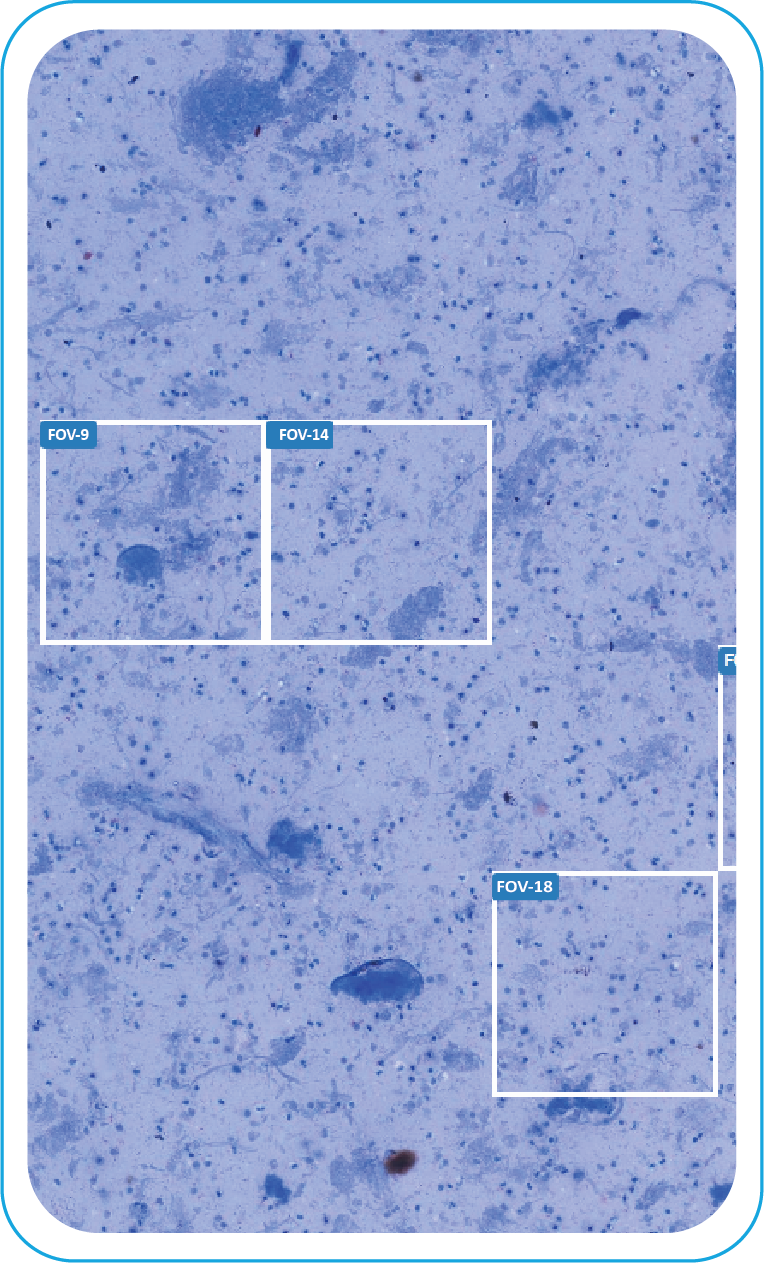
FOV Generated
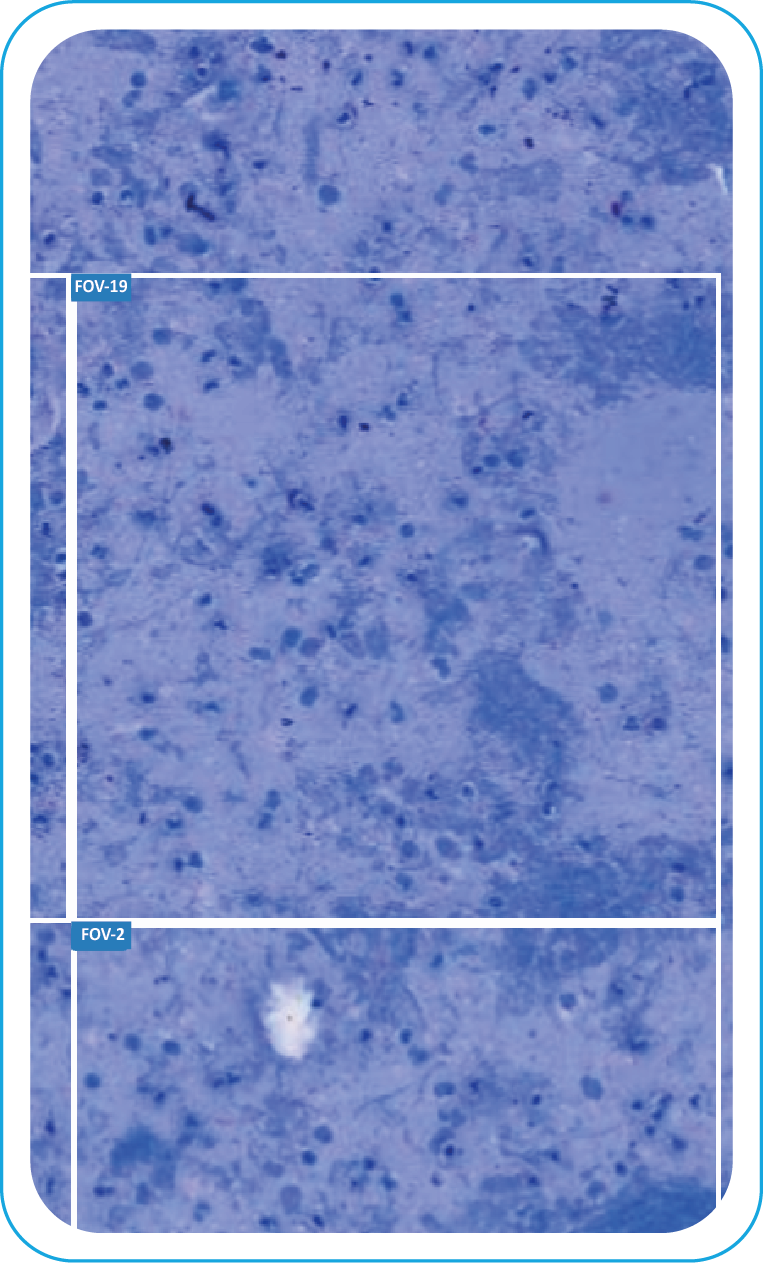
Clear FOV
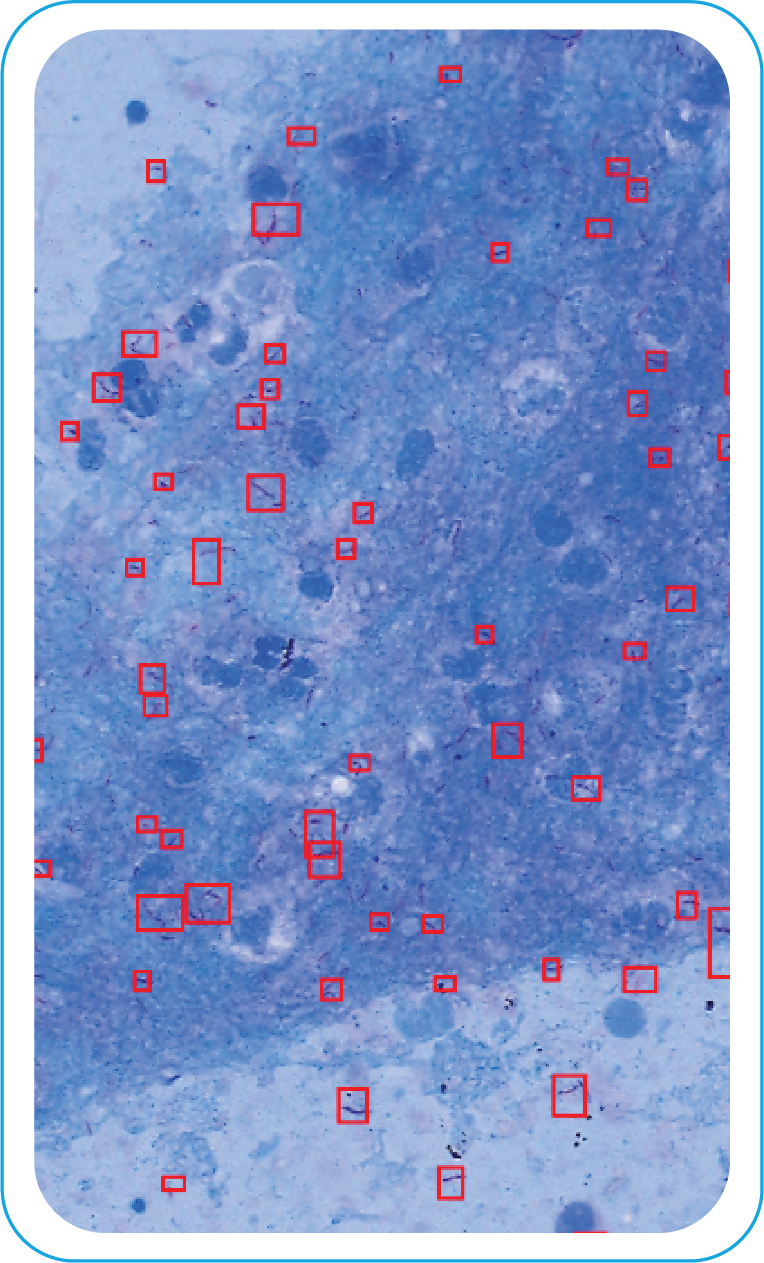
Visible Bacilli
with Annotations
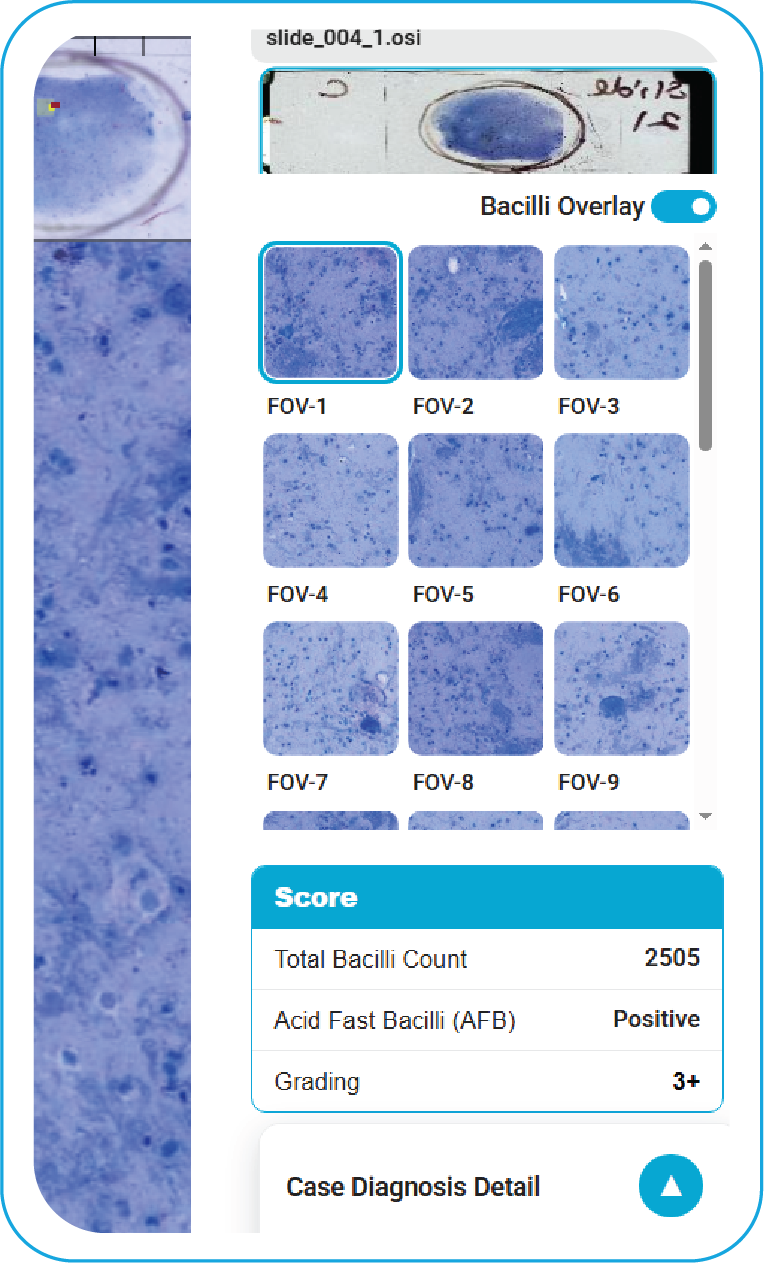
Software Output Panel
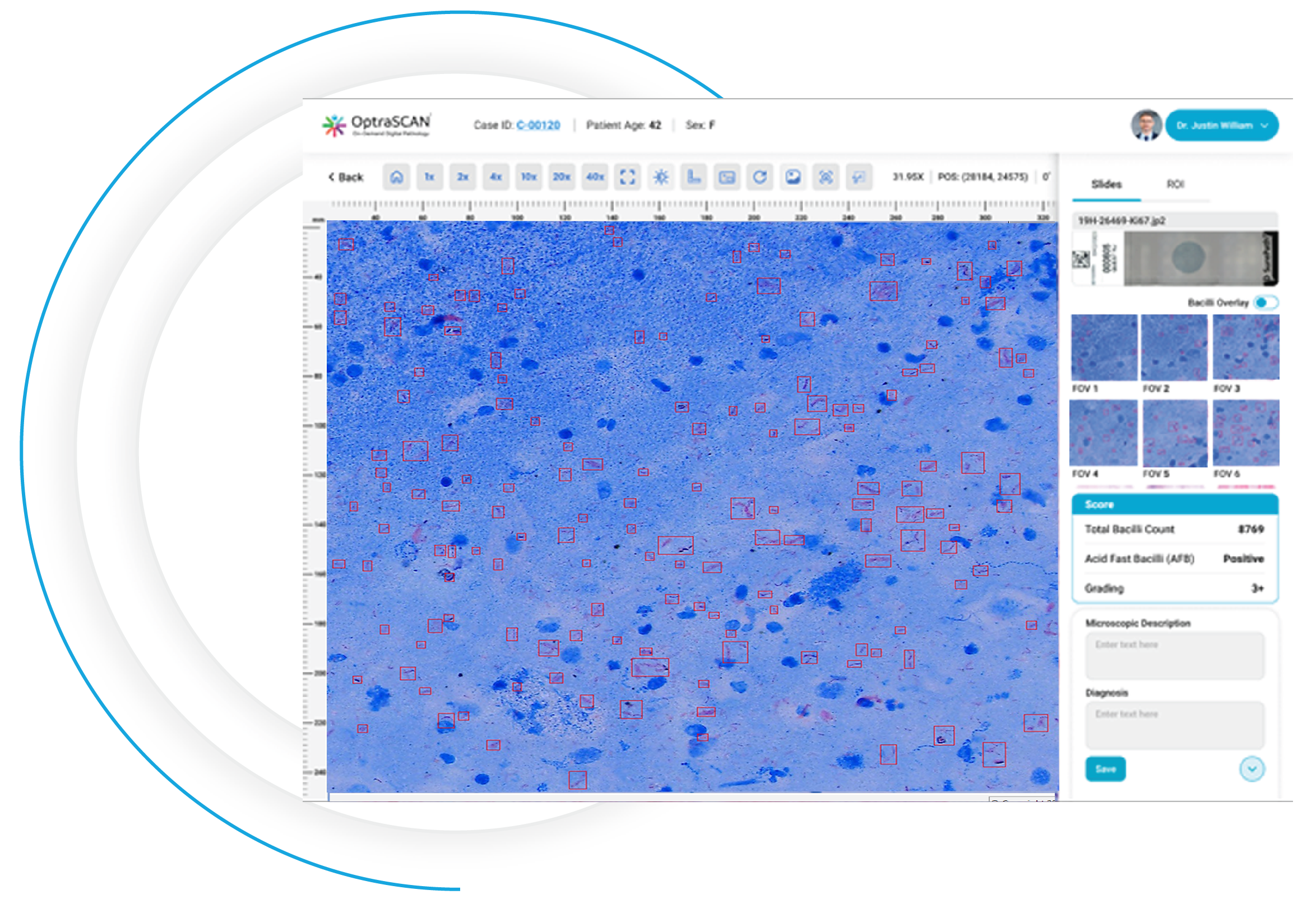
BactoSiA™ is designed to support laboratories managing high testing volumes and repeat evaluations by:



Please fill out the form below and complete all questions with an asterisk (*)